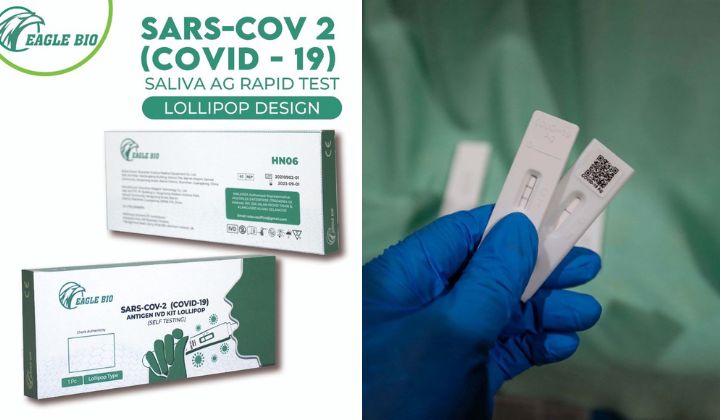
MDA Revokes Conditional Approval For China’s Eagle Bio Self-Test Kit
It’s the third Covid-19 self-test kit from China to have its conditional approval revoked.
Subscribe to our Telegram channel for the latest stories and updates.
The Medical Device Authority (MDA) has revoked the conditional approval for another China-made Covid-19 self-test kit.
According to MDA CEO, Ahmad Shariff, the Eagle Bio SARS-CoV-2 Antigen IVD Kit Lollipop is the third Covid-19 self-test kit to be removed from shelves.
Previously, MDA revoked the conditional approvals of two other self-test kits in April: Deepblue Covid-19 Antigen Test Kit (Colloidal Gold) and Wiz Biotech SARS-CoV-2 Antigen Rapid Test.
MDA would also be sending letters to four other companies this week to demand these companies carry out further investigations and corrective measures on their defective kits.
Ahmad explained that companies are given a chance to further investigate and take corrective measures on the defective test kits.
However, the MDA will revoke the approval of the product if the test kits fail to meet the requirements for the second time.
What makes an effective self-test kit?
Self-test kits must show 100% for specificity, and at least 90% sensitivity before the MDA gives conditional approval, Free Malaysia Today reported.
Sensitivity measures the proportion of positive test results from all truly positive samples. This is to ensure the kit can correctly identify those with the disease (the true positives) while minimising the number of false negative results.
Specificity measures the proportion of negative test results from all truly negative samples. This is so the test kit can correctly identify those without the disease (the true negatives) while minimising false positive results.
As of 19 July, 142 RTK antigen self-test kits have been given conditional approval by MDA.
Share your thoughts with us via TRP’s Facebook, Twitter, and Instagram.