Subscribe to our Telegram channel for the latest stories and updates.
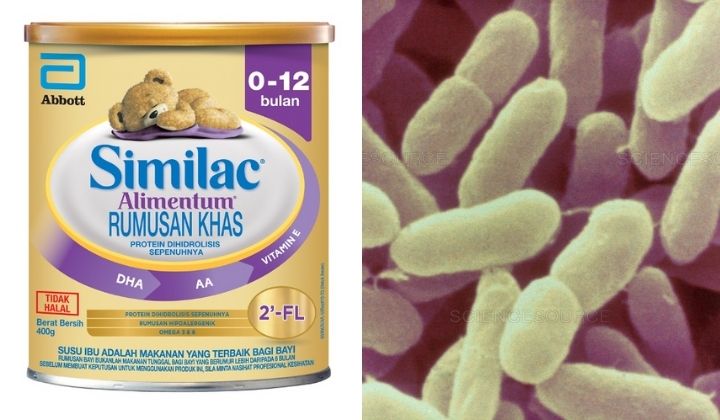
Abbott has initiated a proactive voluntary recall of Similac Alimentum and Similac Human Milk Fortifier that were manufactured in Sturgis, Michigan, USA after receiving reports of bacterial contamination.
READ MORE: KKM Recalls 2 Similac Baby Formulas Due To Bacteria Contamination
In light of Abbott’s recent voluntary recall of both infant formula milk products, the company’s spokesperson has stated that the products distributed in Malaysia did not test positive for the presence of either Cronobacter Sakazakii or Salmonella Newport.
However, the company will continue testing their products.
Both Alimentum and Human Milk Fortifier are the only product range impacted and have been recalled from places where these products are mostly available such as hospitals and pharmacies.
Parents and guardians are advised to talk to their healthcare professional about suitable, alternative feeding options in the meantime.
The Abbott spokesperson added that no other Abbott nutrition products – including Similac – are impacted by the recall and consumers can continue to use these products with confidence.
For any questions on the product recall and return, parents can call Abbott Nutrition Careline at 1800-88-6233 or email at wecare@abbott.com.
Share your thoughts with us via TRP’s Facebook, Twitter, and Instagram.